Research
Vascular Disorder Group
Our group mainly studies cerebral aneurysms that cause subarachnoid hemorrhage and cerebral ischemia that causes cerebral infarction. The following is a brief description of our research.
Computational flow dynamics analysis of cerebral aneurysms
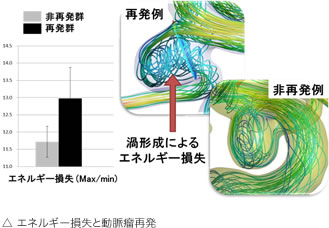
Computational flow dynamics (CFD) analysis is a well-established technique in science and engineering, but it has been applied in various fields in recent years.
In the field of neurosurgery, it is expected to elucidate the mechanism of development, growth and rupture of cerebral aneurysms, and to predict arterial stiffness and recurrence after treatment.
We are investigating recurrence after endovascular coil embolization. Although endovascular coil embolization is minimally invasive and has been widely used in recent years for the treatment of cerebral aneurysms, it has a higher recurrence rate than open-head clipping. Therefore, if we could predict the recurrence after coil embolization, we could change the treatment strategy before surgery, which would be useful in choosing a treatment method for cerebral aneurysms.
We investigated the relationship between various hydrodynamic factors and recurrence and found that the pressure difference (pressure at the coil surface) is strongly related to recurrence, which can be measured in an arterial model based on preoperative examination data and is very Useful factors.
Nambu I, et al., Neurosurgery (2019) 84: 607-615
We are currently conducting further validation, including increasing the number of cases and examining it in different vascular models. We believe that if the veracity of this factor increases, it could be clinically applicable and provide useful information for patients.
Elucidation of the phenomenon of delayed neuronal cell death
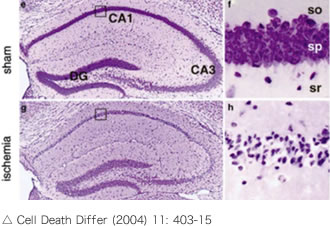
Delayed neuronal death is a phenomenon in which neuronal loss and atrophy (apoptosis) occur within 2-3 days after ischemia in the CA1 region of the hippocampus, as shown in the figure on the right. We hypothesized that intravascular administration of the protein esRAGE could prevent delayed neuronal death.
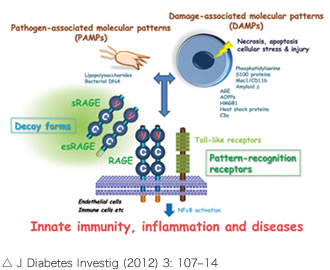
There are two main types of RAGE: membrane RAGE and soluble RAGE (such as esRAGE). Membrane RAGE is a single transmembrane protein with three domains (V, C, and C), and is involved in inflammatory signaling during ischemia through ligand binding to the V domain. On the other hand, esRAGE is a splice variant of membrane-type RAGE that blocks ligand binding to membrane-type RAGE as a competitive receptor.
 The BCCAO model, in which bilateral common carotid arteries of mice are ligated for 20 minutes and reperfused to induce delayed neuronal death in the CA1 region of the hippocampus, is commonly used to produce delayed neuronal death. In the present study, we also used this model to investigate how delayed neuronal death is affected in the CA1 region of the hippocampus with and without intravenous administration of esRAGE. We are studying the effects of esRAGE on delayed neuronal death by measuring the number of neurons in the CA1 region of the hippocampus
The BCCAO model, in which bilateral common carotid arteries of mice are ligated for 20 minutes and reperfused to induce delayed neuronal death in the CA1 region of the hippocampus, is commonly used to produce delayed neuronal death. In the present study, we also used this model to investigate how delayed neuronal death is affected in the CA1 region of the hippocampus with and without intravenous administration of esRAGE. We are studying the effects of esRAGE on delayed neuronal death by measuring the number of neurons in the CA1 region of the hippocampus
and using staining to detect apoptosis (e.g. caspase III staining).
Research on cerebral ischemia and neuroprotection
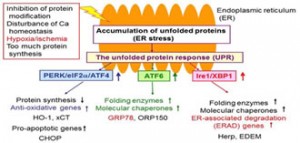 The endoplasmic reticulum is an intracellular organelle responsible for protein and lipid biosynthesis and for maintaining Ca homeostasis. In response to this, cells maintain intracellular homeostasis by activating the unfolded protein response (UPR), a stress response that originates from the endoplasmic reticulum membrane, and improving the intracellular environment.
The endoplasmic reticulum is an intracellular organelle responsible for protein and lipid biosynthesis and for maintaining Ca homeostasis. In response to this, cells maintain intracellular homeostasis by activating the unfolded protein response (UPR), a stress response that originates from the endoplasmic reticulum membrane, and improving the intracellular environment.
It has been reported that cerebral ischemia induces endoplasmic reticulum stress and that activation of the UPR leads to neuroprotection, but the detailed mechanism of this is still unknown. In order to elucidate the mechanism of endoplasmic reticulum stress in cerebral ischemia, we used ATF6α knockout mice.
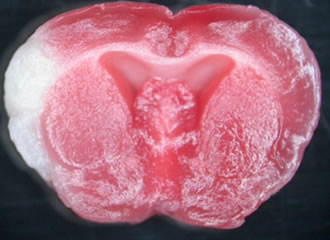
We used the mouse model of permanent middle cerebral artery occlusion (MCAO) and cultured astrocytes to elucidate, among other things, the importance of the major transcription factor ATF6α in the endoplasmic reticulum stress response. The MCAO model is a method of opening the temporal bone of the mouse and coagulating and severing the middle cerebral artery seen directly below with MCAO.
When MCAO was generated in wild-type and Atf6α knockout mice, increased infarct volume and neuronal death were observed in Atf6α knockout mice. Furthermore, the same mice showed reduced activation of astrocytes and glial scar formation and increased tissue damage to non-infarcted foci.
We also found that the expression of phosphorylated STAT3 (p-STAT3) and GFAP, which are associated with glial scar formation, was attenuated in astrocytes lacking Atf6α.
These results indicate that ATF6α plays an important role in neuroprotection through the activation of astrocytes and glial scar formation that occurs under brain ischemia.
Yoshikawa A, et al., J Neurochem (2015) 132: 342-53
ATF6β and whole brain ischemia
As mentioned above, the transcription factor ATF6α has been found to play an important role in neuroprotection via astrocyte activation and glial scar formation that occurs under cerebral ischemia. On the other hand, the role of another subtype of ATF6, ATF6β, in cerebral ischemia remains unclear. Therefore, we are analyzing the involvement of ATF6β in neuroprotection under total cerebral ischemia.
Whole brain ischemia is modeled as bilateral common carotid arteries occlusion (BCCAO) by incising the midline of the neck under anesthesia to expose the bilateral common carotid arteries and then temporarily blocking them with microclips. After creating the model, we are assessing for neuronal cell death and glial cell changes in the hippocampal CA1 region.

