Research
Neuroendoscopy Group
Board certified in neuroendoscopic surgery
In recent years, there has been a dramatic increase in the use of endoscopy in neurosurgery. The Japanese Society of Neurological Endoscopy (http://square.umin.ac.jp/jsne/qualifications.html) launched a technical certification system in 2006. This is a system to certify the basic techniques of neuroendoscopy. We perform a number of surgeries under the guidance of board-certified doctors, such as the following.
Transnasal endoscopic surgery

Simultaneous surgery with nasal and craniotomy
Neuroendoscopic surgery for intra-ventricular and intra-axial lesions
(Indications: hydrocephalus, intracerebral hemorrhage, brain tumor, etc.)
In hydrocephalus, we perform a third ventricular fundus window opening that does not require shunt placement. In addition, we actively perform endoscopic tumor removal and hematoma removal in intracerebral hemorrhage and brain tumors by perforation/small craniotomy instead of conventional open surgery.
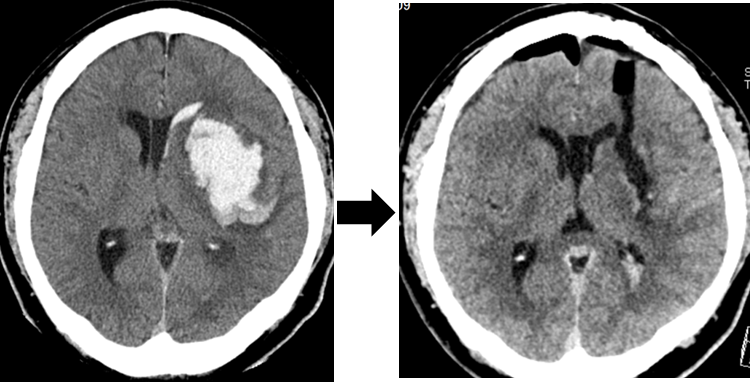
Endoscopic removal of intracerebral hematoma (left: before surgery, right: after surgery)
Research
We focus on the following studies to make use of the results and questions from clinical practice, including the surgery described above.
1) Mechanism of headache in pituitary tumors
Headache is one of the symptoms of pituitary tumor as well as visual field disorder and pituitary dysfunction. However, there is no clear mechanism of headache in this disease. The most probable mechanism of headache development is compression of the nerves in the dura around the tumor or inflammation. Headache induces headache in Rathke’s cyst, a pituitary tumor, due to inflammatory spillover, even if it is small in size. We have shown that appropriate surgery can improve headache (Fukui I et al. World Neurosurg 2017). We also focused on the relationship between intrasellar Turkish sella pressure and headache in pituitary adenomas. We measured intratumoral pressure using a brain pressure sensor during nasal pituitary tumor removal and reported the mechanism of headache along with its correlated results (Hayashi Y et al. Neurosurgery 2019). We aim to conduct future studies that will allow us to predict how much the headache will improve before surgery is performed.
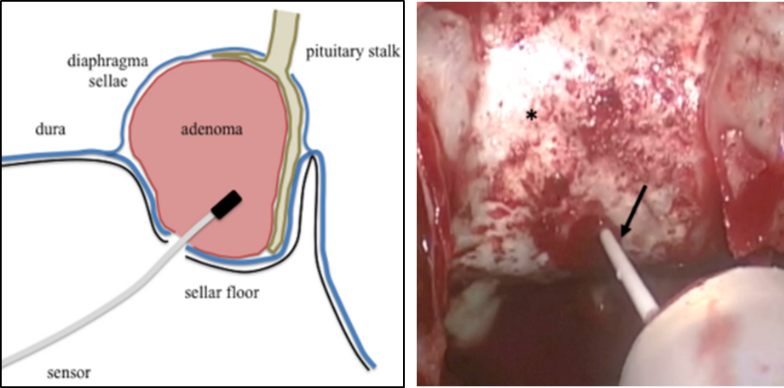
Schematic and intraoperative pressure measurement of intrasellar pressure
(Hayashi Y et al. Neurosurgery 2019)
2) Development and imaging analysis of diabetes insipidus in pituitary tumors
Central diabetes insipidus is one of the symptoms of pituitary tumors. It is also one of the perioperative complications of pituitary tumor removal and is caused by impaired vasopressin secretion from the posterior pituitary gland. Vasopressin is indicated by a high signal in the posterior lobe of the pituitary gland on MRI T1-weighted images. We reported that the morphology of the high signal in the pituitary pattern can predict the time of recovery from diabetes insipidus (Hayashi Y et al. Pituitary 2016). We have also analyzed cases of enuresis in Rathke’s cleft cyst and reported on the characteristics and prognosis of the development of the disease (Oishi M et al. Clin Neurol Neurosurg 2018). We are currently focusing on oxytocin as well as vasopressin, which is secreted by the posterior pituitary gland, and studying its relationship to MRI imaging.
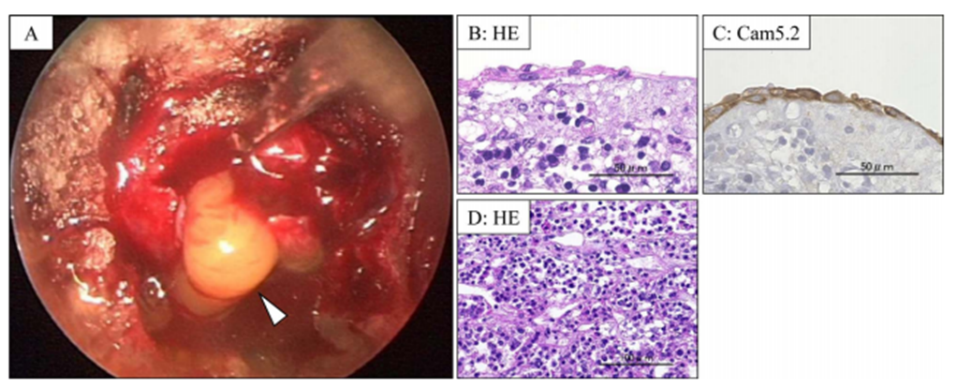
Contents and pathological findings of Rathke’s cleft cyst
(Oishi M et al. Clin Neurol Neurosurg 2018)
3) Clinical features in acromegaly and their impact on surgery
Acromegaly is caused by growth hormone-producing pituitary adenomas and can lead to a variety of complications. We have treated many patients with acromegaly in collaboration with the Department of Endocrinology and Metabolism. We have found the clinical features unique to acromegaly and have reported the usefulness of these features in surgery. We reported on the anatomical features of the internal carotid artery (Sasagawa Y et al. Pituitary 2016) and the pathogenesis of empty sella and its impact on surgery (Sasagawa et al. Pituitary 2017). We have also reported on the clinical features and surgical outcomes of acromegaly in the elderly, which has been increasing in recent years (Sasagawa et al. World Neurosurg 2018). In the future, we are studying not only surgery but also pharmacological treatment of acromegaly and its effects.
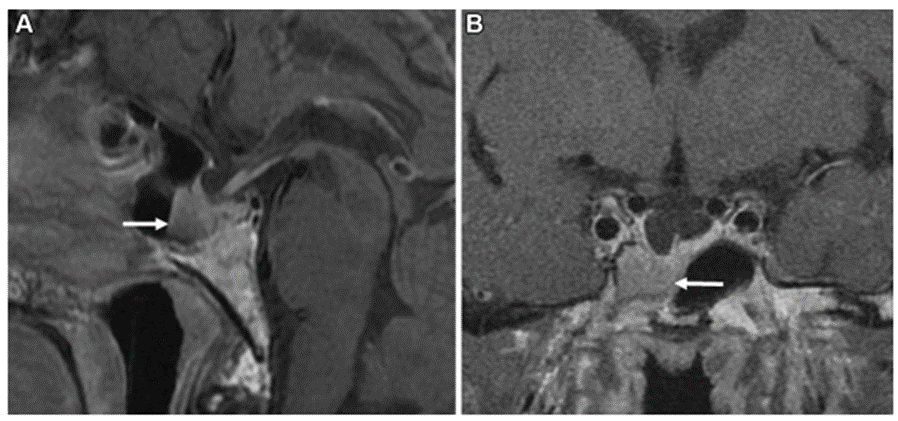
Acromegaly with empty sella
(Sasagawa et al. Pituitary 2017)
4) Development of new neuroendoscopic devices
We are developing medical devices for neuroendoscopic surgery in collaboration with the Faculty of Engineering, Kanazawa University. Especially in endoscopic surgery for brain tumors, it is important to minimize bleeding. We aim to develop an instrument that can detect the beating of blood vessels when the tumor is grasped with forceps, and we report the results of basic experiments (Yokota H et al. Sensors 2019). In the future, we plan to conduct experiments using actual animal blood vessels in an environment that more closely resembles a human organism.
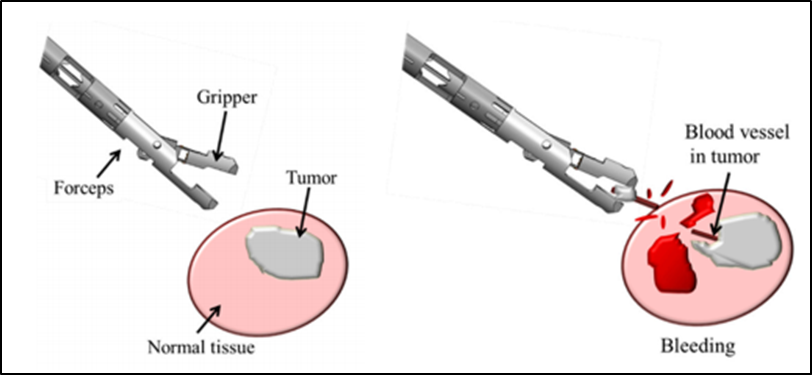
An experiment to detect the beat of a tumor blood vessel when it is grasped
(Yokota H et al. Sensors 2019)

